MediGel® Sucralose is an Efficient Oral Delivery System for Pain Medication
The Animal Welfare Act (AWA) defines (in Section 1.1) painful procedure to any animal as any procedure that would reasonably be expected to cause more than slight or momentary pain or distress in a human being to which that procedure was applied, that is, pain in excess of that caused by injections or other minor procedures. AWA requires (in Section 2.31) that procedures that may cause more than momentary or slight pain or distress to laboratory animals should be performed with appropriate pain relieving drugs and that pre-operative and post-operative medical care should be available and provided as necessary by a qualified veterinarian.
Medication is Required for any Level of Pain
Different levels of pain can be expected, from minimal to severe, depending on the procedures that are being performed. AWA makes a distinction between minor and major operative procedures, with a major operative procedure being define as any surgical intervention that penetrates and exposes a body cavity or any procedure which produces permanent impairment of physical or physiological functions, while a minor operative procedure does not.
Any level of pain requires monitoring and analgesic regimens consisting of local anesthetic, opioid, and NSAID either singly used, or in combination. Carprofen and Meloxicam are the most common NSAIDs used for the management of acute or chronic pain, however the most common route of administration for those medication is by injection, which itself causes distress to the animal. The two studies below assessed MediGel® Sucralose as an efficient delivery method for Carprofen and Meloxicam.
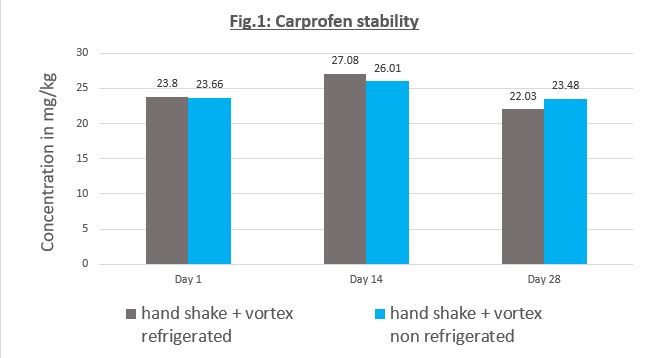
Carprofen is Stable in MediGel® Sucralose
In a pilot study with MediGel® Sucralose, The City of Hope (COH) in California assessed the best protocol to mix Carporfen in MediGel® Sucralose, and tested the sterility and stability of Carporfen in MediGel® Sucralose over a one month period. The results show that Carporfen in MediGel® Sucralose displayed homogeneous mixing, drug stability and sterility out to 28 days when either refrigerated or not. In summary, we recommend to liquify MediGel® Sucralose for 15min at 60C, inject the medication through the foil lid, handshake then vortex the cup each for 15 seconds, then refrigerate for up to one month.
For a thorough protocol, please see our step by step guide or video.
Carprofen and Meloxicam in MediGel® Sucralose Achieve Therapeutic Levels
Using the protocol above, the Institut de Recherches Cliniques de Montréal (IRCM) provided Carprofen or Meloxicam in MediGel® Sucralose to C57BL/6J mice, then assessed plasma concentrations for those two active ingredients. The graphs below show that the plasma concentrations of meloxicam and carprofen were both very stable, increasing over time and reaching therapeutic levels when administered in MediGel® Sucralose, comparable to levels obtained when the drugs are administered by injection.
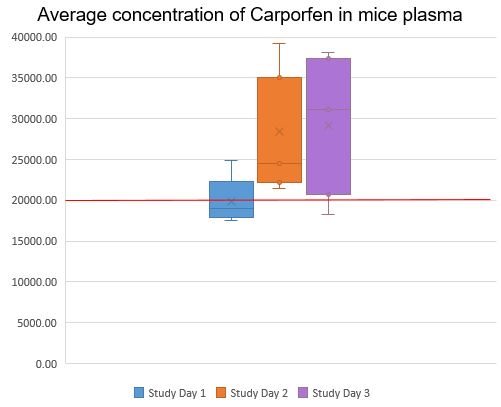
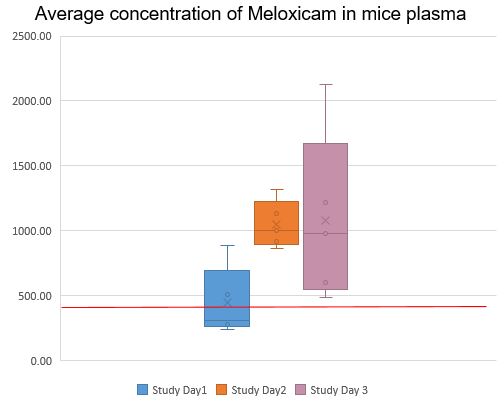
In conclusion, MediGel® Sucralose, when used under the care and supervision of a veterinarian, is a convenient and effective delivery system for pain medication in laboratory mice. To try it for yourself, request a sample
If interested, please review the full studies: City of Hope study for stability and Montreal Clinical Research Institute study for efficiency.