

Cryopreservation as a Risk Mitigation Tool
When working with laboratory rodents, especially with Genetically Engineered Models (GEM), time and money are two valuable resources that you will need. Whether you create the models yourself or get them from a vendor or collaborator, you need to anticipate the resources required for the design and creation of the model, importation of the mice, and establishment of the colony in your vivarium, along with the associated maintenance costs (per diem rate, feed and bedding, enrichment, genotyping, technician time, etc.). And, once you have invested so much in a model, you are still not protected against any sudden loss of your colony from unexpected circumstances, such as:
- Natural disasters: Floods, hurricanes and pandemics have time and time again threatened our homes, jobs and annihilated our furry friends. We can all remember hurricane Sandy (1,2) in NY in 2012, and COVID-19 pandemic last year (3, 4), and the disastrous consequences they had on research animals.
- Facility issues: A sudden power outage or a failure in the ventilation or water systems in your vivarium can cut off life supplies for your animals and lead to a tragic outcome.
- Animal room problems: Most mouse rooms are SPF (specific-pathogen free), meaning that they are routinely tested and demonstrated to be free of a specific list of opportunistic and commensal organisms and disease-causing pathogens that can affect mouse health and research outcomes (5). However, a unwanted pathogen outbreak can decimate the colony, and make the animals unsuitable for many experimental uses (6, 7). To learn more about GF and SPF mice, refer to our Germ Free and Microbiota blog series.
- Colony difficulties: Genetic drift (see below) or breeding issues can go undetected and unbeknownst to you, can slowly infiltrate and destroy your colonies.
All those factors can put you at risk of losing your colony! Think of cryopreservation as a risk mitigation tool for research continuity, and not only a solution during crisis.
Genetic Drift Can Put your Colony at Risk
Let us take a moment to look at genetic drift in more detail (8, 9). Genetic drift is defined as “the constant tendency of genes to evolve even in the absence of selective forces”, meaning that mutations will constantly appear in the genome. Some of those changes might be benign, or disappear, but some might become fixed and spread in your colony and may cause physiological or phenotypic changes, thereby threatening the integrity of your model and significantly impacting your research. Unfortunately, genetic drift is undetectable for a while unless you run complete comparisons of whole genome sequence data, or until you start seeing phenotypic divergences and unreproducible results.
Although genetic drift is a natural selection process and cannot be stopped, you can take some measures to greatly limit its impact in your colonies by following those few tips:
- Maintain pedigree lines and detailed colony records. If changes start to appear, the lineage can be traced and the animals can be taken out of the colony.
- Watch for phenotypic changes, including any breeding performance deviation, in both mutants and controls
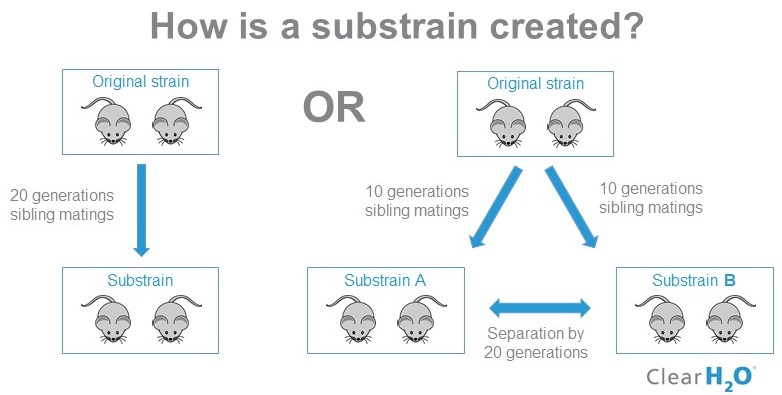
- Refresh breeders frequently : Isolated breeding in your lab might lead to creating a substrain, a strain of mice that has diverged from their parent strain for 20 or more generations of sibling matings (10, 11) (see graph below). Substrains can show functional differences in phenotype and behavior, and can lead to inconsistent data or misinterpretation of results. It is thus recommended to refresh the genetic background and backcross your colony back into the original strain every 10 generations, by bringing new breeders back into the colony (12).
- Avoid selection pressure: Selecting breeders for certain attributes might lead you to fix a mutation in the colony! Instead, choose breeders at random.
- Cryopreserve unique strains (see next paragraph on cell preservation by freezing)
Sperm or Embryo Cryopreservation for Research Continuity
Freezing any living organism is fatal, as the cells are injured by the quick transition of water into ice at low temperatures. However, a cryoprotective agent that is able to penetrate the cells and has low toxicity (such as dimethylsulfoxide (DMSO), ethylene glycol (EG) or glycerol), when mixed with the cell before freezing, can modify the water behavior during the freezing and thawing processes, reduce the freezing injuries to the cells, and preserve the structure of the living cells. This is the basis of cryopreservation.
Cryopreservation (13-15) is defined as the preservation and storage of living cells at extremely cold temperatures, generally in liquid nitrogen at -196C (-320F), indefinitely. In the management of your breeding colonies, this technique allows you to have a backup for strains you don’t want or need on the shelf, for breeder replenishment, or as insurance against unforeseen circumstances that impact your colony. There are two ways to cryopreserve your strains, both with certain advantages depending on the unique characteristics of your strain:
- Sperm cryopreservation: This method is easier and cheaper up-front, some vendors even offer a kit to do it yourself! In addition, this method is ideal for simple colonies such as colonies on an inbred background, strains with only one mutation, or smaller colonies as only two males are needed to do the cryopreservation.
- Embryo cryopreservation: This method needs more animals to start with (10 females and 2 males) and might be more expensive upfront, but is definitely the method of choice for more complicated strains: strains that are homozygous, that have a unique or specific background, or strains with multiple mutations. With embryo cryopreservation, those characteristics are conserved.
Cryorecovery by In Vitro Fertilization (IVF)
Once you are ready to bring your mice and rats back on the shelf, sperm and embryos can be recovered to live mice. In vitro fertilization technology (IVF, 16) allow to create embryos in a petri dish, using the cryopreserved sperm and oocytes from superovulated females (females that have been treated with stimulating hormones (pregnant mare serum gonadotropin (PMSG) and 5 IU human chorionic gonadotropin (HCG) to produce more eggs than normal (17, 18)).
At the two-cell stage, those embryos (or cryopreserved embryos (19)) can be then transferred into pseudo-pregnant females (females that have been mated with vasectomized males, inducing a ‘pregnancy-like’ hormonal response, and making the females receptive to pregnancy). Nutrition can be quite important at this stage: folic acid has been shown to increase sperm count & progesterone levels, while omega-3 fatty acids help improve sperm motility & oocyte quality. Our DietGel® Prenatal contains 49.9 mcg of folic acid and 2.3 g of omega-3s per 100 g of gel, and can be fed to females throughout all stages of the reproductive cycle.

REQUEST YOUR FREE SAMPLE TODAY!
This technique can be used on a large scale to produce hundreds of age-matched pups at once. Alternatively, this method can also be used with fresh samples to rederive a compromised strain, or to rescue an endangered one.
In brief, while cryopreservation and cryorecovery are some very technical methods, requiring highly skilled technicians to perform those tasks, many vendors and core departments offer these services. Cryopreservation can save your strains from many disasters both external and internal to your colonies and should be considered as an essential tool in your colony management plan.
Cryopreservation as a Risk Mitigation Tool – References:
(1) Hurricane Sandy’s Lesser-Known Victims: Lab Rats
(2) Hurricane Sandy: Thousands of rodents drown at NY lab
(3) ‘It’s heartbreaking.’ Labs are euthanizing thousands of mice in response to coronavirus pandemic
(4) Animal Facilities Make Tough Decisions as Pandemic Closes Labs
(5) The difference between GF and SPF mice – The Jackson Laboratory
(6) Natural Pathogens of Laboratory Mice, Rats, and Rabbits and Their Effects on Research
(7) Biology of the Laboratory Mouse – Infectious Diseases
(8) Maybe it’s not you, Maybe it’s your mice – The Jackson Laboratory
(9) Strain background and genetic drift – The Jackson Laboratory
(10) The Laboratory Mouse – Strains, Stocks and Mutant Mice
(11) What’s in a (Sub)strain?
(12) How to refresh your mutant or transgenic mouse strains – The Jackson Laboratory
(13) Cryopreservation Insights – Taconic
(14) Cryopreservation and its clinical applications
(15) Mouse cryopreservation – The Jackson Laboratory
(16) IVF CARD Protocol – Vanderbilt University
(17) Mouse Female Superovulation Guideline – Stanford Medicine
(18) Superovulation Strategies for 6 Commonly Used Mouse Strains
(19) Cryopreservation & Assisted Reproduction Laboratory, transfer of cryopreserved mouse embryos – Frederick National Laboratory for Cancer Research
Featured image from (20)

